How Many More Protons Does Sodium Have Than Oxygen
It has an atomic mass of 229 which is often rounded up to 23 to accurately determine the number of neutrons in the element. The number of neutrons can be found by subtraction of the atomic number from sodiums atomic mass of twenty three.
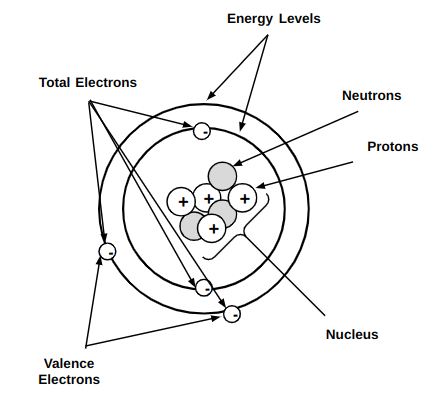
Atoms Periodic Table Science Quizizz
The sodium ion has an extra positive charge shown by the sign.

. An Na ion is a sodium atom that has lost one electron as that makes the number of electrons in the atom equal to that of the nearest Nobel gas Neon which has 10 electrons. How Many More Protons Does Sodium Have Than Oxygen. What Is The Limiting Reagent Between A Reaction Of Hclaq And Naohaq.
13 19 34 12. The sodium atom has 11 protons 11 electrons and 12 neutrons. An oxygen atom for example has eight protons.
The element sodium has 12 neutrons. Its possible because sodium has 11 protons and electrons but neon bass 10 protons and electrons. If an ion has a 2 charge like Zn.
There are 11 protons and 11 electrons in an atom of sodium. The element sodium has 12 neutrons 11 electrons and 11 protons. The number of protons of an atom cannot change via any chemical reaction so you add or subtract electrons to get the correct charge.
How many electrons does the sodium atom have in the first three orbits. It has the same number of protons as electrons. It has one more proton than it does electrons.
On the other hand Na has 11 protons 10 electrons and 12 neutrons. Like What Purpose Does It Serve. The Periodic Table of Elements shows the number of protons for each element.
The oxygen atom has therefore 8 neutrons 8 protons and 8 electrons. The number of neutrons is the atomic weight 2298 in this case rounded minus the number of protons. Neutrons have no electric charges.
How many protons does Neon have. The Mass Of Cobalt-60 In A Sample Is Found To Have Decreased From 0800g To 0200g In A Period Of 105 Years. From This Information What Is The Half-life Of Cobalt.
Oxygen Has electron configuration of 1s2 2s2 3p4 Identify each of the following as an example of a chemical change or a physical change. An anion carries a negative charge and has more electrons than protons. Therefore sodium has 11 protons 11.
If oxygen has 8 total electrons how many more electrons does hydrogen need to become stable. How many electrons are present in a Phosphorus 2 atom. There are also 12 neutronsif stable in the nucleus combinedwith the protons with electrons circling the neutron.
This tells us that sodium has 11 protons and because it is neutral it has 11 electrons. How is it possible that sodium has more volume than neon when neon has more valence electrons. Moisture in the air forms beads of liquid water on a cold window pane yeast cells in bread make carbon dioxide and ethanol from sugar and a reactant decomposes to form tow products.
Since the weight of the protons and neutrons is about 1 the electrons weigh almost nothing compared to protons and neutrons and we know we have 8 protons in the nucleus we can calculate the number of neutrons by subtracting 8 from 16 16 - 8 which equals 8. Neutrons do not have a net electric charge so the number of neutrons does not matter in the calculation. It has one more electron than it does protons.
The number of protons in an atom determines what the element is. The element also has an atomic number of 11 meaning that it has 11 electrons and 11 protons. The sodium ion still has 11 protons 11 positive charges but now only 10 electrons 10 negative charges.
The mass number of an element tells us the number of protons AND neutrons in an atom the two particles that have a measurable mass. Similarly how many protons neutrons and electrons does sodium have. Given the data calculated in parts a b c and d determine the initial rate for a reaction that starts with 085 m of reagent a and 070 m of reagents b and c.
Sodium has a mass number of 23amu. The number of electrons and protons come from the elements atomic number which is same 11. How many electrons does sodium have.
Nitrogen and oxygen sodium and chlorine hydrogen and argon. All group 1 metals will form a 1 ion when they react with non-metals.

Infographic Database Infographic Examples For Your Inspiration Chemistry Classroom Chemistry Lessons Teaching Chemistry

Atomic Model Cards Atom Model Atom Model Project Atom Project

Atom Neon 3d Model Atom Model Atom Model Project Neon Atom Model

How Atoms Work Bohr Model Atom Model Project Atom Model

Tetraborate Borax Ion Structure Pink Boron Red Oxygen White Hydrogen This Tetrameric Boron Structure Comprises Two Boro Boron Borax Sodium Tetraborate

See The Electron Configuration Diagrams For Atoms Of The Elements Electron Configuration Atom Diagram Electrons

2012 In Infographics How Graphic News Saw The World Boson De Higgs Fisica De Particulas Ciencias De La Naturaleza

Protons Neutrons Electrons Modelos Atomicos Atomo Ciencias Naturais

Molecules And Compounds Reading Comprehension Passage And Questions Printa In 2021 Science Reading Comprehension Reading Comprehension Passages Comprehension Passage

Pin By Rosmery On School Projects Atom Model Atom Model Project Neon Atom

Schematic Diagram Of Carbon Atom Atomic Theory Atom Diagram Atom

Boron 3d Atomic Model Chemistry Projects Science Projects Atom Model

Element Word Search Sample Answers Answer Key Answer Key Worksheets Map Activities Answer Keys

Bohr Atomic Model H20 3d Jewelry Set Hydrogen And Oxygen Etsy Science Models Physical Science Experiments Physical Science

Atom Project 13 14 Chemistry Tutorials Practice And Demonstrations Atom Project Teaching Chemistry Atom Model

If You Have A School Project To Make A 3d Model Of The Sodium Atom You Can Use Several Materials You Simp Science Models High School Science Class Atom Model

Chemistry Articles Buzzle Com Teaching Chemistry Chemistry Basics Chemistry Classroom

Science For Homeschool Bright Ideas Press Atom Model Project Chemistry Projects

Pin By S Borda On Killzone Atom Model Project Atom Atom Model
Comments
Post a Comment